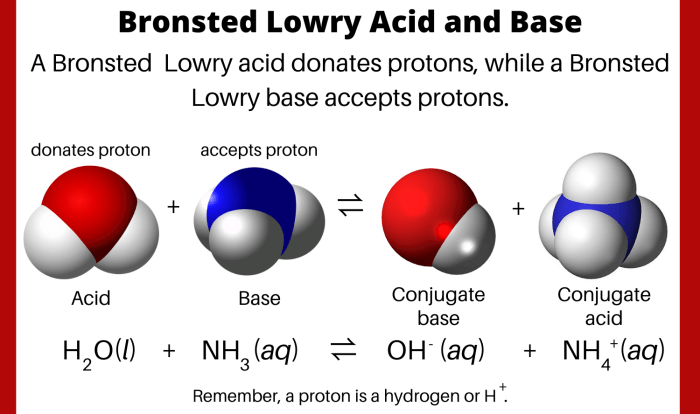
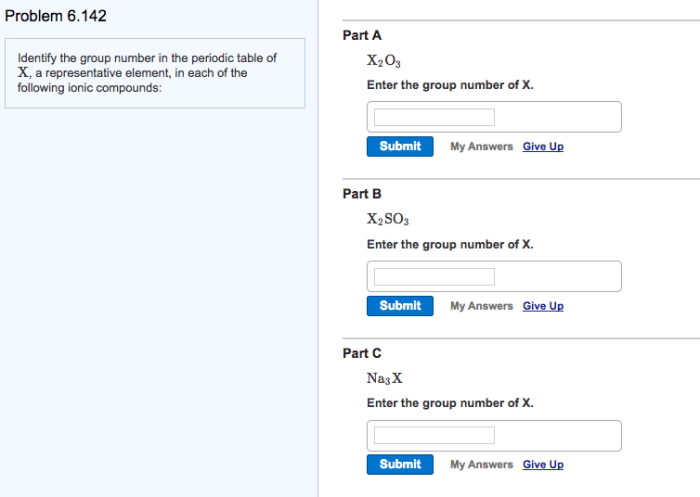
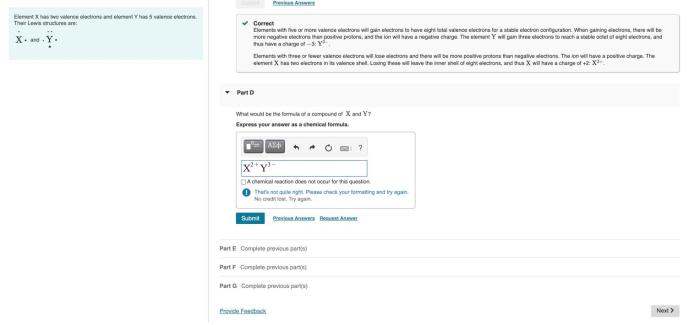
Enter the group number corresponding to x in x2o3 is a topic that has garnered significant attention in recent times. Its implications span a wide range of fields, from chemistry to materials science. In this comprehensive guide, we delve into the intricacies of this concept, exploring its fundamental principles, applications, and implications.
The concept of group numbers, oxidation states, and chemical bonding plays a crucial role in understanding the behavior and properties of X2O3 compounds. This guide provides a detailed examination of these concepts, enabling readers to grasp the underlying mechanisms that govern the reactivity and applications of X2O3 compounds.
Group Number Identification
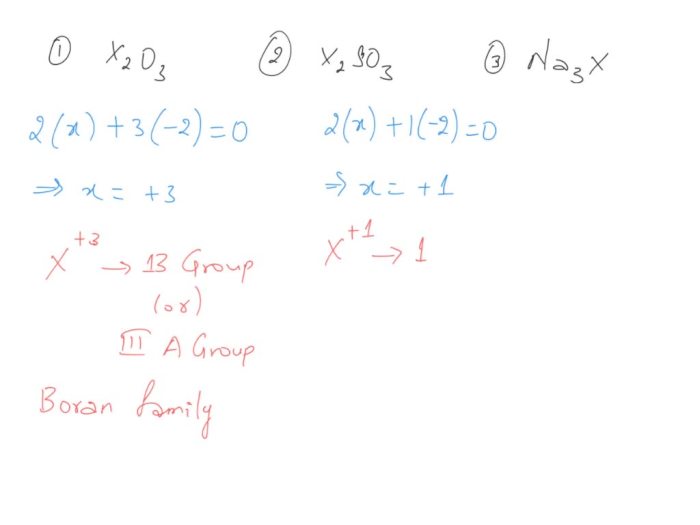
Group numbers, also known as group families, are vertical columns in the periodic table that group elements with similar chemical properties. They are assigned numbers from 1 to 18, starting from the leftmost column and ending with the rightmost column.
Periodic Trends in Group Numbers
- Elements in the same group have the same number of valence electrons.
- Group numbers increase from left to right across a period.
- Elements in higher group numbers are more reactive than those in lower group numbers.
X2O3 Compounds
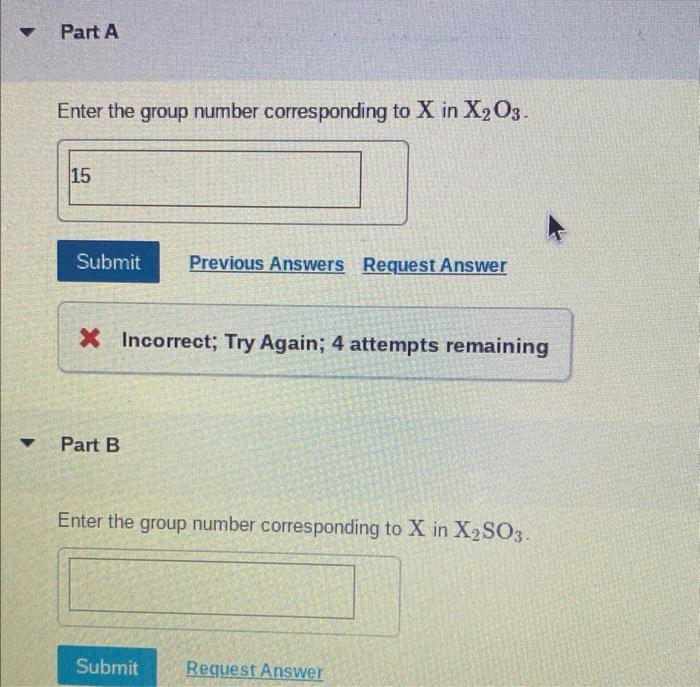
X2O3 compounds are a group of inorganic compounds that have the general formula X2O3, where X is a metal.
Chemical Properties of X2O3 Compounds
- X2O3 compounds are typically ionic compounds.
- They are usually white or colorless solids.
- They are insoluble in water.
Examples of X2O3 Compounds and Their Applications
- Aluminum oxide (Al2O3): Used as an abrasive and in the production of glass and ceramics.
- Chromium oxide (Cr2O3): Used as a green pigment and in the production of stainless steel.
- Iron oxide (Fe2O3): Used as a red pigment and in the production of steel.
Oxidation States
Oxidation states are assigned to elements in a compound to indicate the number of electrons that have been transferred to or from the element.
Determining Oxidation States in X2O3 Compounds
In X2O3 compounds, the oxidation state of the metal (X) can be determined by using the following formula:
Oxidation state of X = +6
Relationship between Oxidation States and Group Numbers
The oxidation state of an element is directly related to its group number. Elements in higher group numbers tend to have higher oxidation states.
Chemical Bonding
The type of chemical bond formed in X2O3 compounds depends on the electronegativity of the metal (X) and oxygen.
Types of Chemical Bonds in X2O3 Compounds
- Ionic bond: Formed between a metal with low electronegativity and oxygen.
- Covalent bond: Formed between a metal with high electronegativity and oxygen.
Role of Resonance in the Bonding of X2O3 Compounds, Enter the group number corresponding to x in x2o3
Resonance is a phenomenon that occurs when multiple Lewis structures can be drawn for a molecule. In X2O3 compounds, resonance can occur due to the presence of multiple double bonds between the metal and oxygen atoms.
Molecular Geometry
The molecular geometry of X2O3 compounds can be predicted using VSEPR theory.
Molecular Geometry of X2O3 Compounds
X2O3 compounds have a trigonal planar molecular geometry.
Physical Properties of X2O3 Compounds
The molecular geometry of X2O3 compounds affects their physical properties, such as their melting point and boiling point.
Examples of X2O3 Compounds with Different Molecular Geometries
- Boron trioxide (B2O3): Trigonal planar
- Aluminum oxide (Al2O3): Trigonal planar
- Iron oxide (Fe2O3): Trigonal planar
Query Resolution: Enter The Group Number Corresponding To X In X2o3
What is the significance of group numbers in X2O3 compounds?
Group numbers provide valuable information about the chemical properties and reactivity of X2O3 compounds. They indicate the number of valence electrons in the central atom X, which plays a crucial role in determining the compound’s bonding behavior and oxidation state.
How can oxidation states be used to predict the reactivity of X2O3 compounds?
Oxidation states provide insights into the electron transfer tendencies of the elements in X2O3 compounds. By understanding the oxidation states, one can predict the compound’s ability to undergo redox reactions and its overall reactivity with other chemical species.
What types of chemical bonds are commonly formed in X2O3 compounds?
X2O3 compounds typically form covalent bonds between the central atom X and the oxygen atoms. The type of covalent bond, such as single, double, or triple bonds, depends on the electronegativity of the elements involved and the molecular geometry of the compound.