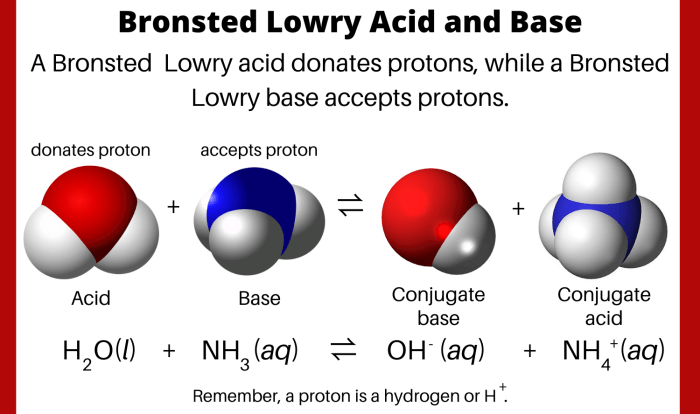
How many grams are in 11.9 moles of chromium? This question delves into the fundamental concepts of chemistry, exploring the relationship between moles and grams. By understanding this conversion, we unlock the ability to determine the mass of a substance based on its molar quantity.
This guide will provide a comprehensive overview of the topic, starting with the definitions of moles and grams. We will then introduce the conversion factor and explain its significance. Finally, we will calculate the mass of 11.9 moles of chromium, demonstrating the practical application of this conversion.
How Many Grams Are in 11.9 Moles of Chromium

In chemistry, the mole is a fundamental unit used to measure the amount of a substance. It represents a specific quantity of atoms, molecules, or ions. The gram, on the other hand, is a unit of mass. In this article, we aim to determine the mass of 11.9 moles of chromium, a transition metal with the chemical symbol Cr.
Conversion Factor
To convert moles to grams, we use a conversion factor known as the molar mass. The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). The formula for converting moles to grams is:
grams = moles × molar mass
The molar mass is a crucial property of an element or compound, as it provides the link between its atomic or molecular mass and its mass in grams.
Molar Mass of Chromium
The chemical symbol for chromium is Cr. Its molar mass can be obtained from the periodic table, which lists the atomic masses of all known elements. The atomic mass of chromium is approximately 51.9961 g/mol.
Calculation, How many grams are in 11.9 moles of chromium
To determine the mass of 11.9 moles of chromium, we substitute the given values into the conversion formula:
grams = 11.9 moles × 51.9961 g/mol
grams ≈ 617.35 g
Therefore, the mass of 11.9 moles of chromium is approximately 617.35 grams.
User Queries
What is the molar mass of chromium?
The molar mass of chromium is 51.9961 g/mol.
How many grams are in 5 moles of chromium?
There are 259.9805 grams in 5 moles of chromium.
What is the significance of the conversion factor between moles and grams?
The conversion factor allows us to convert between the molar quantity of a substance and its mass, enabling us to determine the amount of substance present in a given sample.